Wikimedia commons
While Hollywood loves to imagine humans encountering all manner of horrific monsters in the depths of space, the greatest threat to a long-term, manned space mission may not come with tentacles, or extra mouths, or an insatiable love for human flesh. It may, in fact, be the invisible microbes that hitch a ride with us from Earth.
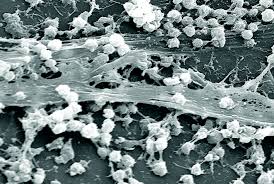
Every day it seems, we become increasingly aware of our insignificance on planet Earth when compared with our microbial cousins. Our bodies host vast ecosystems of bacteria, often said to outnumber our own cells ten to one (I looked for a citation on that one, and curiously, couldn’t link the statement back to a single peer-reviewed article, so take it as you will). So, it shouldn’t come as a surprise that when humans go into space, we bring loads of our invisible comrades along with us. And some of them, it turns out, actually like it up there, forming biofilms that gunk up our water filtration systems and degrade the very fabric of our ships. But these bacteria are more than just a nuisance: They may pose significant health threats to astronauts. That’s why researchers at NASA and other space agencies recently published a series of papers on them in the journal Microbes and Environments. These review papers outline what we know about microbes in spaceships, and the specific measures space agencies are taking to monitor, counteract and control biological contamination.
Space agencies, including NASA, the European Space Agency (ESA) and the Japan Aerospace Exploration Agency (JAXA), routinely monitor the microbes on manned spacecrafts. Most monitoring efforts involve collecting samples from various parts of the indoor environment (air and water samples, surface swabs) culturing microbes on board, and brining them back to the ground for identification. Some of the most common microbes found on spacecrafts are human commensals—stuff we carry with us on our skin— including bacterial genera Staphylococcus, Bacillus and Micrococcus, and fungal genera Penicillum, Apsergillus and Cladosporium. This shouldn’t come as a surprise; recent studies from across a range of indoor environments also find microbial communities filled with critters that lives on us (for instance, see my post on this recent study about the NYC subway microbiome).

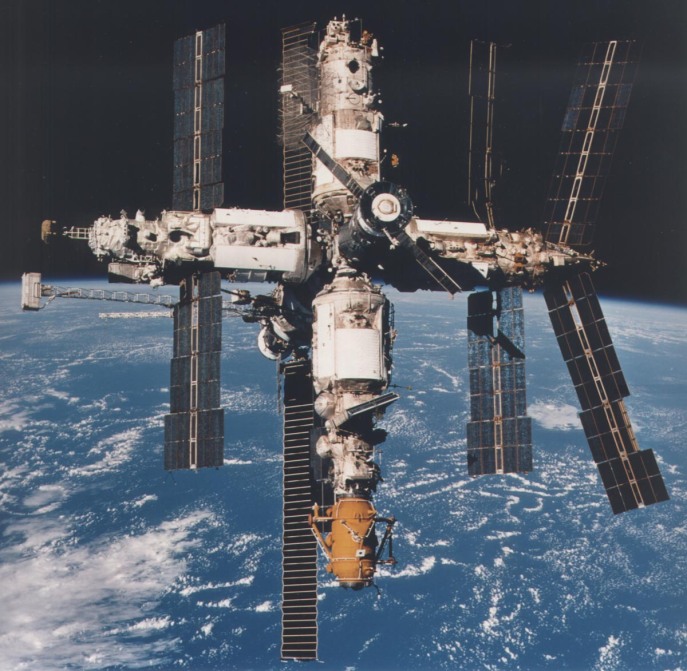
International Space Station, NASA
What’s more concerning than the mere presence of microbes on spacecrafts is the fact that some bugs become tougher after a stint in space. Various researchers have observed increased growth and biofilm formation, and cell wall thickening, in strains of Eschericia coli, Pseudomonas aeruginosa, and Staphylococcus aureus — all potential human pathogens. Often, these changes are correlated with increased antibiotic resistance. Several defining characteristics of the indoor space environment— including microgravity, increased exposure to cosmic radiation, and vacuum— may be important selective forces driving these changes.Unfortunately, human physiology also responds to the space environment: For reasons that are still being studied, astronauts often exhibit decreased immune function following prolonged trips into space.
Given the possibility of the bugs we bring into space becoming more pathogenic, while our own physiologies make us more susceptible to illness, what steps should space agencies be taking to ensure the safety of our astronauts? It’s clear that swabbing for bugs, waiting for them to grow, and IDing them on the ground—methods that have provided us a wealth of useful information about space microbes—isn’t going to cut it for long-term manned missions to Mars, during which astronauts may have to respond to a pathogenic threat in real time:
“As we move farther away from Earth and deeper into the frontier of space, the development of new microbial monitoring technologies aimed at detecting, quantifying, and identifying the presence of target organisms of interest will become increasingly important,”– Dr. Nobuyasu Yamaguchi and colleagues in their recent review paper.
NASA is currently developing new tools that astronauts can use in-flight to rapidly detect microbial pathogens. These include both molecular (DNA based) identification tools and simple portable systems. For instance, a recent proof-of-concept-study found that gold nanoparticles, tagged with an antibody that has a high affinity for S. aureus, could indicate the presence of the bacteria via a colorimetric reaction in less than ten minutes. The JAXA, meanwhile, is developing small microfluidic devices in which bacteria can be detected via a fluorescent dye. In another recent microbial monitoring experiment, the JAXA introduced a handheld particle counter that could detect bacteria-sized particles in the air and store up to 500 measurements.

Mars, Wikimedia commons
There’s abundant circumstantial evidence to suggest that bacterial endospores, as well as some fungal and Archaeal species, may be able to survive an interplanetary journey. Ultimately, the success of long-term manned missions is going to depend on our ability to keep astronauts healthy. We’re just at the frontier of exploring the microbial ecology of indoor environments, and the indoor space environment presents a host of unique environmental challenges. As the technology for monitoring, IDing and responding to pathogenic threats on space ships continues to grow and improve, we move ever-closer to long-term manned space missions becoming a reality.
Three recent review papers covering the microbial ecology of indoor space environments are freely available here:
https://www.jstage.jst.go.jp/article/jsme2/advpub/0/advpub_ME2903rh/_article
https://www.jstage.jst.go.jp/article/jsme2/advpub/0/advpub_ME14032/_article
https://www.jstage.jst.go.jp/article/jsme2/advpub/0/advpub_ME14031/_article
More context on the microbiology research currently conducted by NASA can be found here: http://nasa.gov/exploration/library/esmd_documents.html