Deep in the heart of the Ecuadorian Amazon lies one of the worst environmental disasters in human history. Over the past several decades, oil companies have discharged more than 18 billion gallons of petroleum contaminated wastewater into the Sucumbíos region in northeastern Ecuador. The contamination, which spans a geographic region roughly the size of Rhode Island, is described by the media as “Amazonian Chernobyl.”
Three years ago, an Ecuadorian court ruled that Chevron should pay $18 billion US dollars of damages for the pollution in Sucumbíos. However, the oil giant has so far refused to pay remediation costs, and a bitter legal battle continues to rage.
Now a grassroots effort known as the Amazon Mycorenewal Project (AMP) hopes to take remediation into their own hands- and to the people of Sucumbíos. The scientists, international volunteers and Ecuadorians driving the AMP believe the key to remediation lies in microorganisms thriving in petroleum-contaminated soils. Today, the AMP launched an indiegogo campaign to raise money for research that will determine whether naturally occurring bacteria, fungi and plants can be used to degrade the toxic petro-waste that has plagued the region.
Bioremediation, or using microbes to clean up our environmental messes, is not a new concept. Many microorganisms have been deployed across the world to degrade a range of environmental pollutants, including PCBs, gasoline, radioactive waste and mercury. Several years ago, a group of undergraduate researchers from Yale University visited the Amazon and discovered fungi that eat polyurethane plastic, a synthetic, petroleum-derived material. The enzymes microbes use to degrade synthetics are probably used in nature to decompose lignocellulose, the stuff that makes wood “woody”.
What makes the AMP unique is their ecological approach to bioremediation. By culturing bacteria and fungi that already thrive in petroleum-contaminated environments, the AMP hopes to develop communities of bioremediators that are “naturally suited” to their habitat. Preliminary studies conducted over the last several years show that local fungi, grown in the lab on petroleum-enriched substrates, develop increased resistance to petroleum toxicity.

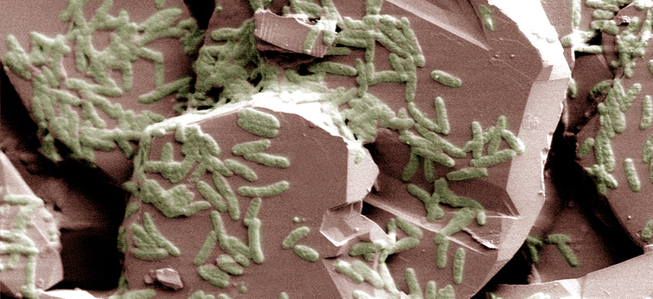
Cultures of “petrophiles”, wild fungi isolated from petroleum contaminated soils in the Ecuadorian Amazon
The other unique aspect of AMP’s approach is the organization’s integration with local communities. Mia Maltz, a fungal ecologist and PhD student at the University of California, Irvine, has been working with the AMP since 2007. Over the last seven years Mia and other members of the AMP have taught mushroom cultivation and mycoremediation (bioremediation using fungi) techniques to locals. By designing and teaching low-cost bioremediation strategies, AMP is empowering locals to do bioremediation on their own.
“A lot of what we’re doing is very low-tech, easy for local people to do” says Mia. “That’s really the goal here. We want things to be inexpensive and cost-effective. The science here is very simple- encouraging the natural process of lignocellulose decomposition. We want to show people the simplicity of what’s happening, help them internalize it, so they can tap into a process that’s a huge part of the web of life.”
This summer, a team of scientists and volunteers will work in Sucumbíos to identify and cultivate microorganisms capable of degrading different hydrocarbons present in petroleum. In collaboration with scientists in the United States, the AMP hopes to use metagenomic analyses to profile entire microbial communities and document key microbial taxa and genes involved in petroleum degradation.
Ultimately, AMP aims to develop inexpensive biofiltration systems. Biofiltration is the process of filtering contaminated water through living organisms (microbes and plants) which detoxify and remove pollutants. The AMP envisions a biofiltration system consisting of several chambers containing different assemblages of petro-degrading bacteria and fungi. After microbial filtration, the wastewater will be fed to plants that can tolerate high concentrations of heavy metals and other residual contaminants.
AMP volunteers are currently developing prototype biofiltration systems. “We want these to be modular and flexible” says Mia. “We plan on licensing everything in the creative commons to make our technology accessible to local communities around the world.”
The Amazon Mycorenewal Project brings together a broad range of expertise to tackle this enormous pollution challenge. Co-founder Bob Rawson is president of two bioremediation companies (International Wastewater Solutions and the Pseudonym Corporation) and has over 36 years of experience cleaning up contaminated soils. Joanna Zlotnik, program director for AMP, has worked as an environmental geologist for ten years, conducting site assessments and doing remediation work. Collaborator Tradd Cotter is the founder and director of Mushroom Mountain LLC. Tradd brings over 22 years of experience in commercial and experimental mushroom cultivation and mycoremediation research.
Check out the Amazon Mycorenewal Project indiegogo campaign and make a contribution here.